

Law 2: A cool transparent gas in front of a hotter source of a continuous spectrum creates a dark line or absorption spectrum. This was seen with flame tests of solid objects and with stars (of which the composition was unsure at the time) Law 1 : Hot opaque body or hot dense gas produces a continuous spectrum: He summarized his findings in three laws: Kichhoff's experimentation showed a connection between absorption and emission spectra.

There seemed to be three main types of spectra: continuous, emission, and absorption. Next - a look at Gustav Kirchhoff's laws and the different types of spectra. The 1868 solar eclipse brought the discovery of the element Helium in the sun's spectrum. They then discovered a couple of new ones - Cesium and Rubidium. Thus the science of spectroscopy was born.īy 1859, Bunsen and Kirchhoff identified all the known element's spectra. They discovered spectral lines - bright, thin lines of color on a dark background. Gustav Kirchhoff teamed up with Bunsen and suggested sending the colors produced in a flame test through a prism. Robert Bunsen (Bunsen burner fame) invented a new gas burner which produced a clean flame - no color of its own. Flame tests of different metals were a well known phenomena. Today we know of 30,000 "Fraunhofer" lines!Ībout 50 years later, chemists also produced dark lines in spectra in the laboratory. He was surprised to find over 600 dark lines crossing this rainbow of light made by the sun. He was a German master optician who used his skills to actually magnify the spectrum the sun produced. In 1814, Joseph von Fraunhofer looked at the light passed through a prism from the sun in a new manner. These continuous spectra became the subject of study as scientists explored blackbody radiation. Scientist found they could make a similar spectrum in the laboratory by heating solid matter and passing the light produced through a prism. The rainbow of colors produced by passing sunlight through a prism is a continuous spectrum. This showed that white light itself was made up of all the colors. During the 1600's, Isaac Newton took the colors made by a prism and passed it through a second prism, recombining the light back to white. The common theory at the time was that the prism itself added the color. But what causes the lines in other types of spectra? How do these lines provide information about these distant objects?Ī rainbow can be produced by passing sunlight through a prism. Studying spectral lines in astronomy provides evidence about the chemical composition and movement of distant objects.Ī blackbody will give a continuous spectrum. Spectroscopy is the systematic study of spectra and spectral lines. The spectrum of an object is a graph of the intensity of radiation vs wavelength (or frequency). Blackbody Radiation and What the Stars Tell Us Electromagnetic Waves - A Sea of InformationĢ.

But what causes the lines in the other types of spectra? Can these lines help in the understanding of the properties of the matter responsible for the various spectra?Ītomic and molecular behavior causing these spectra. A blackbody will give a continuous spectrum.
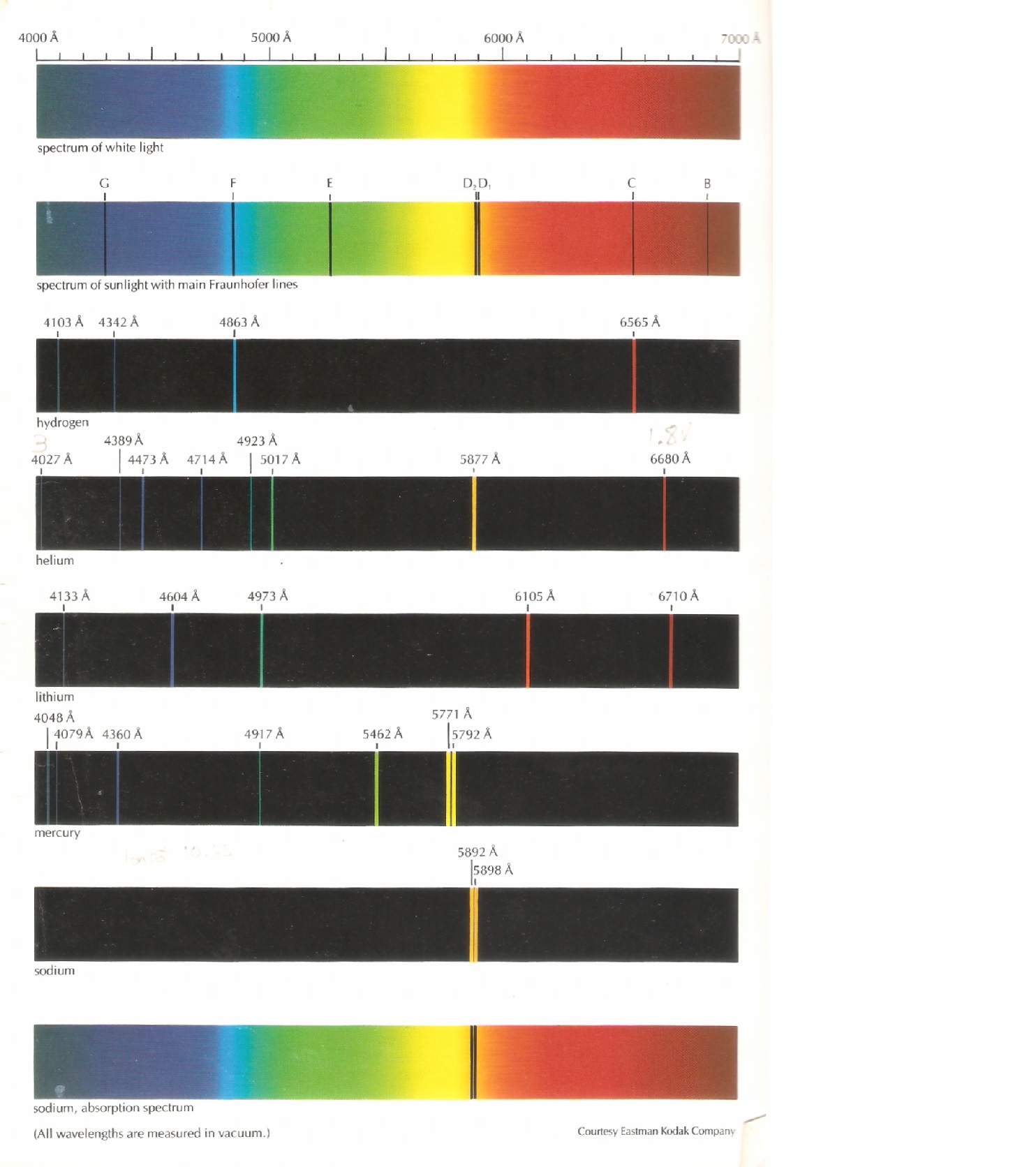
Summary: The spectrum of an object is a graph of the intensity of radiation vs wavelength. Spectral analysis of electromagnetic waves from space. Grade (Age) level: High School (ages 14-18), University Written by: Kathy Gustavson July 2016 Updated July 2022


 0 kommentar(er)
0 kommentar(er)
